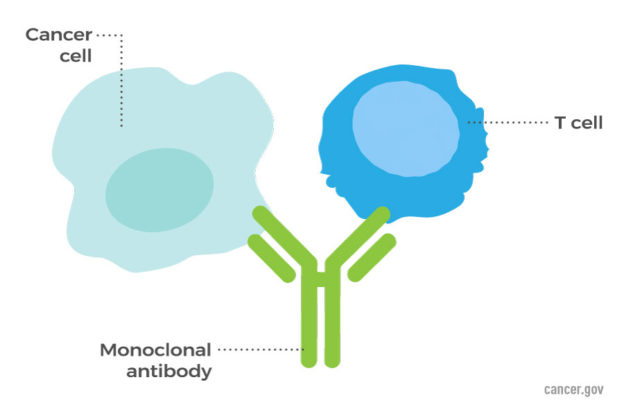
Immunotherapy has resulted in great achievements in cancer treatments. However, significant adverse side effects have also occurred, often resembling autoimmune diseases.
Harnessing the immune system to treat cancer has long been a dream of researchers and oncologists. After overcoming many obstacles, success seems to have been achieved. An essential feature of the immune system is its ability to distinguish “self,” the body’s own cells and tissues, from “non-self,” originating from outside the body. Cancer cells are derived from normal body cells, so the immune system must somehow recognize them as “non-self” to attack them. Although there is ample evidence that the immune system detects abnormalities in cancer cells in the early stages, the cancer cells have developed many methods to evade the immune system. Immunotherapy, such as the use of checkpoint inhibitors is based on stimulating the immune system to detect and destroy cancer cells. Immunotherapy employs unique molecules present on the surface of cancer cells.
The development of autoimmunity is likely an inherent risk in immunotherapies. Considerable effort is underway to unravel the intricacies of adverse immune reactions to predict when adverse events are likely to occur. New therapies should minimize adverse events while maintaining the effectiveness of checkpoint inhibitors.
Improving immunotherapy strategies can involve identifying differences in antibodies present in autoimmunity resulting from immunotherapy with antibodies present in conventional autoimmunity. also, differences in quantities and types of immune cells can be observed between the two types of autoimmunity. This knowledge can be useful in choosing appropriate immunotherapy regimens.
Individual differences are important, as some individuals may have a genetic predisposition for autoimmunity. The unique composition of the individual’s microbiome may impact the development of autoimmunity by altering the immune composition of the tumor and its microenvironment. This immune composition may alter immune responses to immunotherapy treatments.
REFERENCES
1. Couzin-Frankel, Jennifer. “Researchers tackle vexing side effects of potent cancer drugs.” Science. 2022 Sept. 2; 377 (6610): 1028-1029.
2. Weinmann, Sophia and David Pisetsky. “Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitorts.” Rheumatology. 2019:58: vii59-vii67.
3. Young, Arabella, Zoe Quandt and Jeffrey Bluestone. “The balancing act between cancer immunity and autoimmunity in response to immunotherapy.” Cancer Immunol Res. 2018 Dec; 6 (12): 1445-1452.